
A world-first study by Monash University, in Melbourne, Australia has discovered a pathway to the regeneration of insulin in pancreatic stem cells, a major breakthrough toward new therapies to treat Type 1 and Type 2 diabetes.
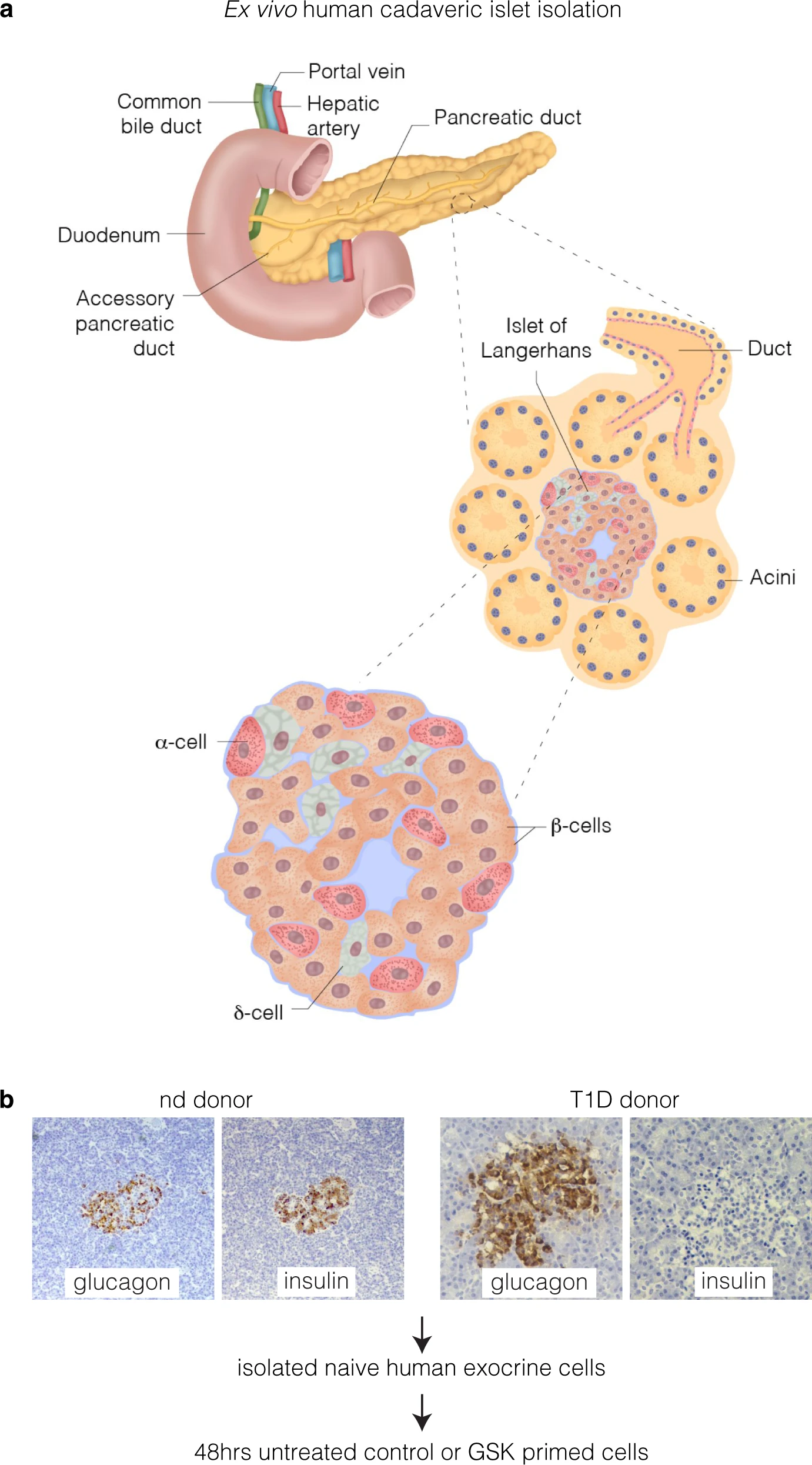
Using the pancreas stem cells of a type 1 diabetic donor, researchers were able to effectively reactivate them to become insulin-expressing and functionally resemble beta-like cells through the use of a drug approved by the US Food and Drug Administration but not currently licenced for diabetes treatment.
Though it requires further work, in principle the new approach would allow insulin-producing cells (beta-cells) that are destroyed in type 1 diabetics to be replaced with newborn insulin generating cells.
The study, led by diabetes experts Professor Sam El-Osta, Dr. Keith Al-Hasani and Dr. Ishant Khurana, from the Monash Department of Diabetes, may lead to a potential treatment option for insulin-dependent diabetes which is diagnosed in seven Australian children every day resulting in a lifetime testing of blood glucose and daily insulin injections, to replace the insulin no longer produced by a damaged pancreas.
As the number of cases of diabetes worldwide approaches 500 million, researchers are scrambling for a limited pool of treatments with unclear effectiveness.
“We consider the research novel and an important step forward towards developing new therapies,” Professor El-Osta said. To restore insulin expression in a damaged pancreas, the researchers had to overcome a series of challenges since the diabetic pancreas was often thought to be too damaged to heal.
The findings are now published in the Nature journal, Signal Transduction and Targeted Therapy
According to Professor El-Osta, by the time an individual is diagnosed with Type 1 diabetes much of their pancreatic beta cells, which produce insulin, have been totally destroyed. These studies show the “diabetic pancreas is not incapable of expressing insulin” and the proof-of-concept experiments “address unmet medical needs in type 1 diabetes”.
The advances in the genetics of diabetes have brought a “greater understanding and along with it a resurgence of interest in the development of potential therapies,” said Professor El-Osta.
“Patients rely on daily insulin injections to replace what would have been produced by the pancreas. Currently, the only other effective therapy requires pancreatic islet transplantation and while this has improved health outcomes for individuals with diabetes, transplantation relies on organ donors, so it has limited widespread use,” said Professor El-Osta.
Co-author of the study, Dr. Al-Hasani says that as we face a globally ageing population and the challenges of escalating numbers of Type 2 diabetes which is strongly correlated with increases in obesity, the need for a cure for diabetes is becoming more urgent,” said Dr Al-Hasani. “Before you get to patients, there are many issues to be resolved,” Dr. Al-Hasani said. ” More work is required to define the properties of these cells and establish protocols to isolate and expand them”, he added. “I would think therapy is pretty far away, however, this represents an important step along the way to devising a lasting treatment that might be applicable for all types of diabetes.”
Professor El-Osta, Drs. Al-Hasani and Khurana have developed a revolutionary method to regenerate insulin cells without the ethical concerns that are commonly associated with embryonic stem cells.

