New research has unlocked insights into how “good fat” tissue could potentially be harnessed to combat obesity and remove glucose from the blood, helping to control diabetes. Published today in Science Advances, the work is a collaboration between researchers with the Perelman School of Medicine at the University of Pennsylvania and University of Cambridge, Free University of Brussels and University of East Anglia.
Human bodies consist of two types of fat: brown and white. Brown fat breaks down blood sugar (glucose) and fat molecules, generating heat in response to cold temperatures helping to maintain normal body temperature. The majority of fat in humans is white fat, and building up too much white fat contributes to obesity and other health issues.
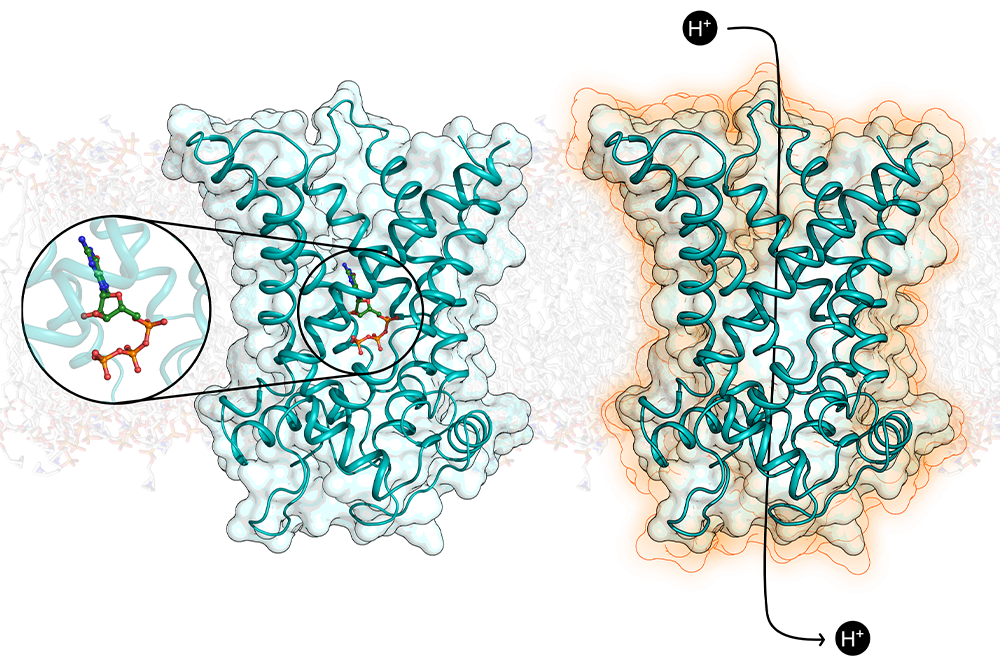
Using the Krios G3i, a cryogenic electron microscope at the Penn Singh Center for Nanotechnology researchers were able to view mitochondrial uncoupling protein 1 (UCP1)—a protein which allows fat tissue to burn off calories as heat—in atomic detail for the first time. This work uncovered new insights into how this protein’s activity in brown fat cells could potentially be harnessed for weight loss.
“This is an exciting development that follows more than four decades of research into what UCP1 looks like and how it works,” said Vera Moiseenkova-Bell, PhD, a professor of Systems Pharmacology and Translational Therapeutics and faculty director of the Beckman Center for Cryo-Electron Microscopy. “These new indings would not have been possible without the collaboration between everyone involved.”
In mammals, UCP1 gives brown fat a specialized ability to burn calories as heat for maintaining a stable body temperature. When activated by fatty acids, UCP1 short-circuits the mitochondria—known as the powerhouse of the cell for its work releasing energy from food—by allowing protons to seep across mitochondrial inner membrane. The short-circuiting generates heat and allowing the body to regulate its temperature through a process called thermogenesis.

