Scientists led by Sabine Taschner-Mandl, PhD, St. Anna Children’s Cancer Research Institute, and Nikolaus Fortelny, PhD, Paris Lodron University of Salzburg, are the first to analyze bone marrow metastases from childhood tumors of the nervous system using modern single-cell sequencing analysis. It turns out that cancer cells prevent cells in their environment from fighting the tumor – a process that could be reversed with medication. The findings were published in the renowned journal Nature Communications.
Neuroblastoma is the most common solid tumor in infants and young children. Despite constantly improving therapy options, more than half of the patients with a very aggressive form (high-risk neuroblastoma) still suffer from relapses. “We specifically studied bone marrow metastases because recurrences often originate there. The tumor cells seem to manipulate their environment so that it supports their growth instead of fighting them,” explains Sabine Taschner-Mandl, Head of the Tumor Biology Group at St. Anna Children’s Cancer Research Institute (St. Anna CCRI).
How cancer cells manipulate their neighboring cells
The recently published study therefore examined the cell architecture and cell-cell communication of neuroblastoma metastases of two major genetic subtypes (MYCN amplification or ATRX mutations) and those without such changes using single-cell transcriptomics and epigenomics. “Until now, only primary tumors have been studied in such detail, but not neuroblastoma metastases,“ says Irfete Fetahu, PhD, co-first author as well as co-corresponding author of the study and postdoc in the Tumor Biology Group.
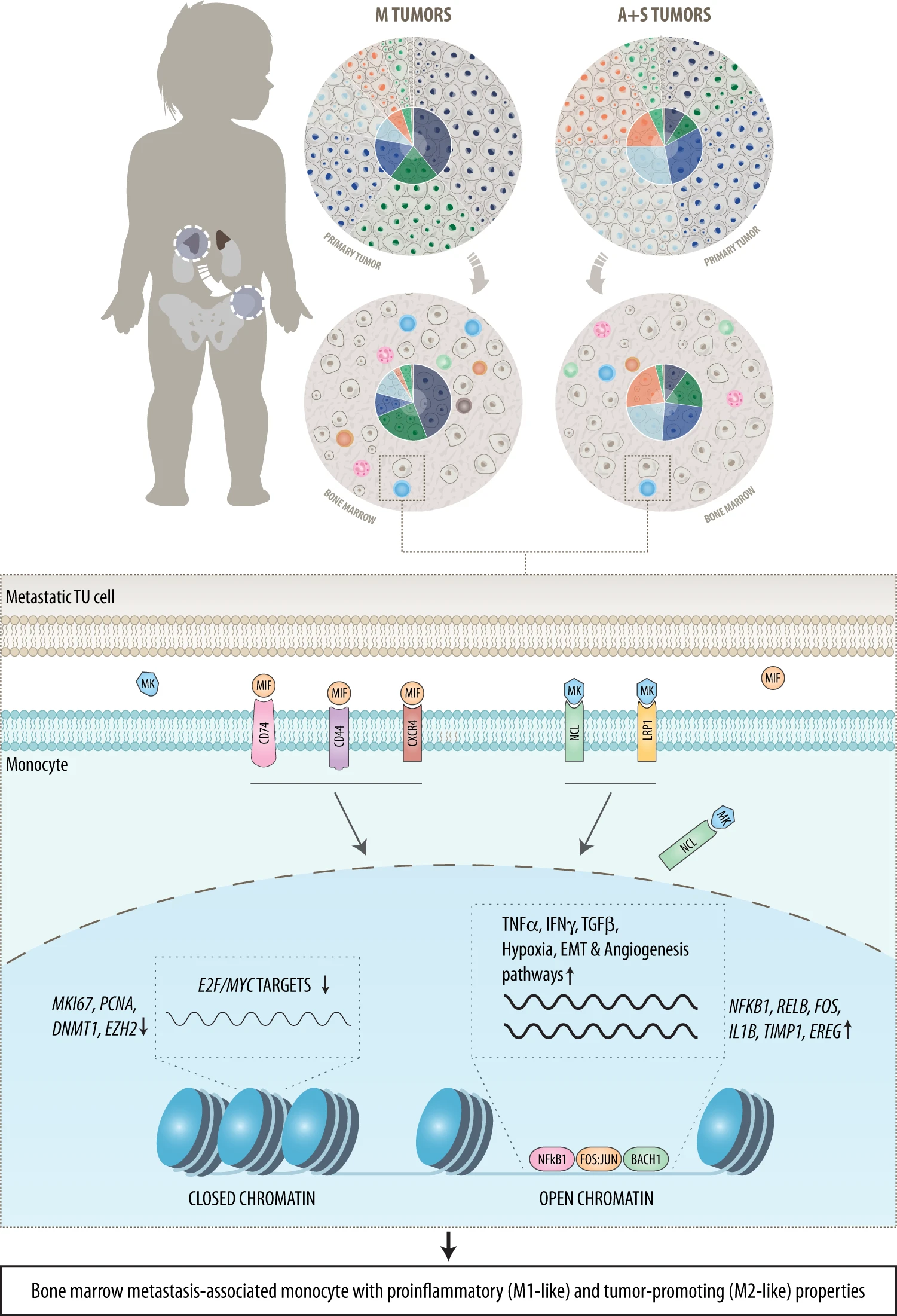

The team examined the interaction of metastatic tumor cells with healthy bone marrow cells in more detail. “We developed algorithms that enabled us to analyze different cells in the bone marrow as well as to model their interactions,” emphasizes Fortelny, head of the Computational Systems Biology Group, Paris Lodron University of Salzburg. “Our analysis has shown that certain cells, so-called monocytes, react to unwanted invaders. In the course of this, they foster growth processes and release cytokines that stimulate tumor growth,” explains Fetahu. Interestingly, investigations at the epigenetic level showed that although monocytes in the tumor microenvironment are activated to attack cancer cells, they are unable to respond appropriately to these signals. “These monocytes receive contradictory messages. As a result, they are no longer able to fight the tumor,“ Fetahu explains the dilemma.
Interfere with pathological immune cell states
The communication between neuroblastoma cells and bone marrow or monocytes is to a large extent regulated by the proteins MK (midkine), MIF (macrophage migration inhibitory factor) and associated molecules. Signaling pathways controlled by these proteins are upregulated in immune cells. “Drugs targeting MK and MIF disrupt this pathological interaction and are currently under investigation. Through selective inhibition, it could be possible to return these pathologically altered monocytes to their original state,“ says Taschner-Mandl.
Metastases act differently
The scientists also found that cellular plasticity, i.e. the ability of cells to change depending on environmental influences, is retained during metastasis. In addition, the gene expression of metastatic tumor cells depends on the neuroblastoma genetic subtype. For example, neuroblastoma cells that have a MYCN amplification only slightly change when they metastasize from the primary tumor to the bone marrow, whereas tumor cells with ATRX mutation show pronounced differences upon metastasis. “The genetics of the tumor lead to characteristic signals and thus very specific changes in the microenvironment of the bone marrow, which is expressed in individual signatures,” says Taschner-Mandl. “This could explain why neuroblastoma patients with ATRX mutations often respond poorly to therapy.”

