
Imlunestrant, an investigational next-generation selective estrogen receptor degrader (SERD), improved progression-free survival in patients with endocrine therapy-pretreated, ER-positive, HER2-negative advanced breast cancer—as monotherapy in patients with ESR1 mutations and as combination therapy with abemaciclib (Verzenio) in all patients, regardless of ESR1 mutation status—according to results from the phase III EMBER-3 clinical trial presented at the San Antonio Breast Cancer Symposium (SABCS), held December 10-13, 2024.
The results of this study were simultaneously published in the New England Journal of Medicine.
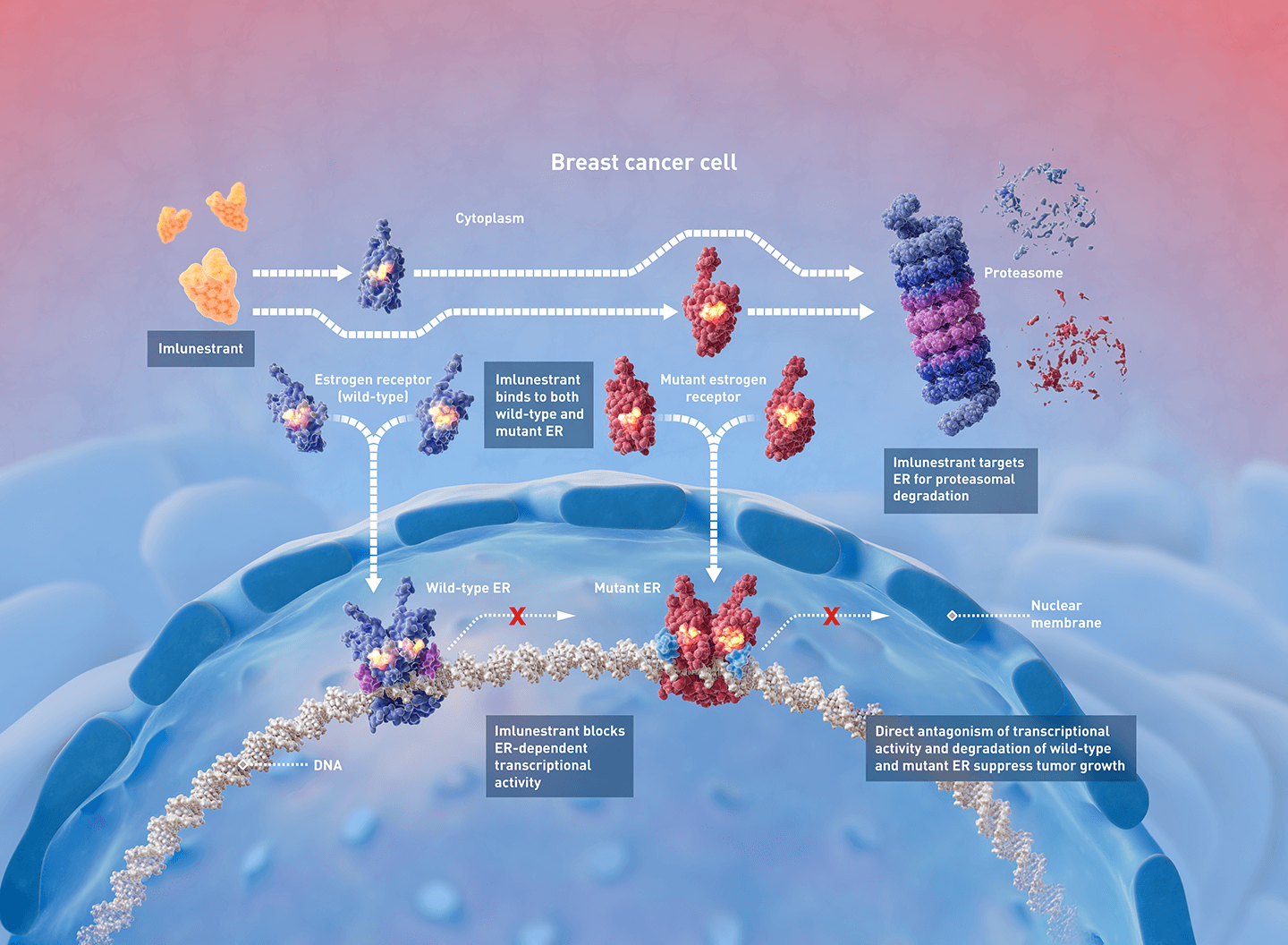
“The main purpose of this EMBER-3 trial was to evaluate the role of imlunestrant, a brain-penetrant oral SERD, as monotherapy or in combination with the CDK4/6 inhibitor, abemaciclib, in patients with ER-positive, HER2-negative advanced breast cancer whose disease had recurred or progressed during or after an aromatase inhibitor alone or with a CDK4/6 inhibitor,” said Komal Jhaveri, MD, the Patricia and James Cayne Chair for Junior Faculty, section head of the Endocrine Therapy Research Program, and clinical director of the Early Drug Development Service at Memorial Sloan Kettering Cancer Center, and presenter of the study.
“CDK4/6 inhibitors have been a critical addition to standard endocrine therapy with aromatase inhibitors, and if progression occurs, combining a SERD with a CDK4/6 inhibitor can be beneficial. However, given the limitations of existing SERDs like fulvestrant, including lack of oral bioavailability and need for monthly intramuscular injection, along with limited efficacy in patients who develop ESR1-mutations, novel SERDs such as imlunestrant are being developed with the goal of improving both efficacy and patient experience through ease of administration,” said Jhaveri, noting that ESR1 mutations are seen in 40%-50% of patients progressing on endocrine therapy.
Unlike fulvestrant, imlunestrant can be taken orally and can penetrate the blood-brain barrier, offering the possibility of targeting central nervous system metastases, explained Jhaveri, who is also an associate professor at Weill Cornell Medical College.
In the multicenter study, 874 patients with ER-positive, HER2-negative advanced breast cancer were randomly assigned (1:1:1) to receive either imlunestrant alone, standard endocrine therapy (fulvestrant or exemestane) alone, or imlunestrant plus abemaciclib.
Compared to standard endocrine therapy, imlunestrant significantly improved progression-free survival in patients with ESR1 mutations but not in the overall population. In the patients harboring ESR1 mutations, imlunestrant reduced the risk of progression or death by 38%.
“These promising results mean that imlunestrant is potentially another single-agent option for the many patients whose recurrent breast cancers harbor ESR1 mutations,” said Jhaveri.
Furthermore, compared to imlunestrant alone, imlunestrant and abemaciclib together were associated with a 43% reduction in risk of progression or death, resulting in median progression-free survival of 9.4 months and 5.5 months respectively, for the combination and monotherapy groups. The benefit of combining these two therapies, both of which can be taken orally, were observed in all patients, including those with and without ESR1 mutations or PI3K-pathway mutations and including those previously treated with a CDK4/6 inhibitor.
“The consistency of these results across clinically relevant subgroups is reassuring given most patients eligible for second-line therapy have received a CDK4/6 inhibitor previously and many currently available second-line therapies require biomarker selection,” noted Jhaveri, who called the data practice changing.
Imlunestrant was well tolerated, both as monotherapy and in combination, Jhaveri said. Imlunestrant showed favorable safety profile, with generally low-grade and manageable adverse events, and without the oral SERD-specific safety signals of ocular or cardiac issues. The safety profile of the combination was consistent with the known fulvestrant-abemaciclib profile with a relatively low discontinuation rate of 6.3% that compares favorably to available combination regimens. Additionally in patient-reported outcomes data from the study, 72% of patients receiving fulvestrant reported injection site pain, swelling, or redness.
“Taken together, these data are encouraging for patients and show that imlunestrant, as monotherapy or combined with abemaciclib, could provide an all-oral targeted therapy option after progression on endocrine therapy for patients with ER-positive, HER2-negative advanced breast cancer.”
One limitation of the study is that unlike the phase III postMONARCH and EMERALD trials, EMBER-3 did not require prior treatment with a CDK4/6 inhibitor. However, most patients (65%) in the combination therapy comparison in EMBER-3 were CDK4/6 inhibitor pretreated and the treatment effect of imlunestrant-abemaciclib in these patients was consistent with the overall population.

