
In a recent study of the brain’s waste drainage system, researchers from Washington University in St. Louis, collaborating with investigators at the National Institute of Neurological Disorders and Stroke (NINDS), a part of the National Institute of Health (NIH), discovered a direct connection between the brain and its tough protective covering, the dura mater. These links may allow waste fluid to leave the brain while also exposing the brain to immune cells and other signals coming from the dura. This challenges the conventional wisdom which has suggested that the brain is cut off from its surroundings by a series of protective barriers, keeping it safe from dangerous chemicals and toxins lurking in the environment.
“Waste fluid moves from the brain into the body much like how sewage leaves our homes,” said NINDS’s Daniel S. Reich, M.D., Ph.D. “In this study, we asked the question of what happens once the ‘drain pipes’ leave the ‘house’—in this case, the brain—and connect up with the city sewer system within the body.” Reich’s group worked jointly with the lab of Jonathan Kipnis, Ph.D., a professor at Washington University in St. Louis.
Reich’s lab used high-resolution magnetic resonance imaging (MRI) to observe the connection between the brain and body’s lymphatic systems in humans. Meanwhile Kipnis’s group was independently using live-cell and other microscopic brain imaging techniques to study these systems in mice.
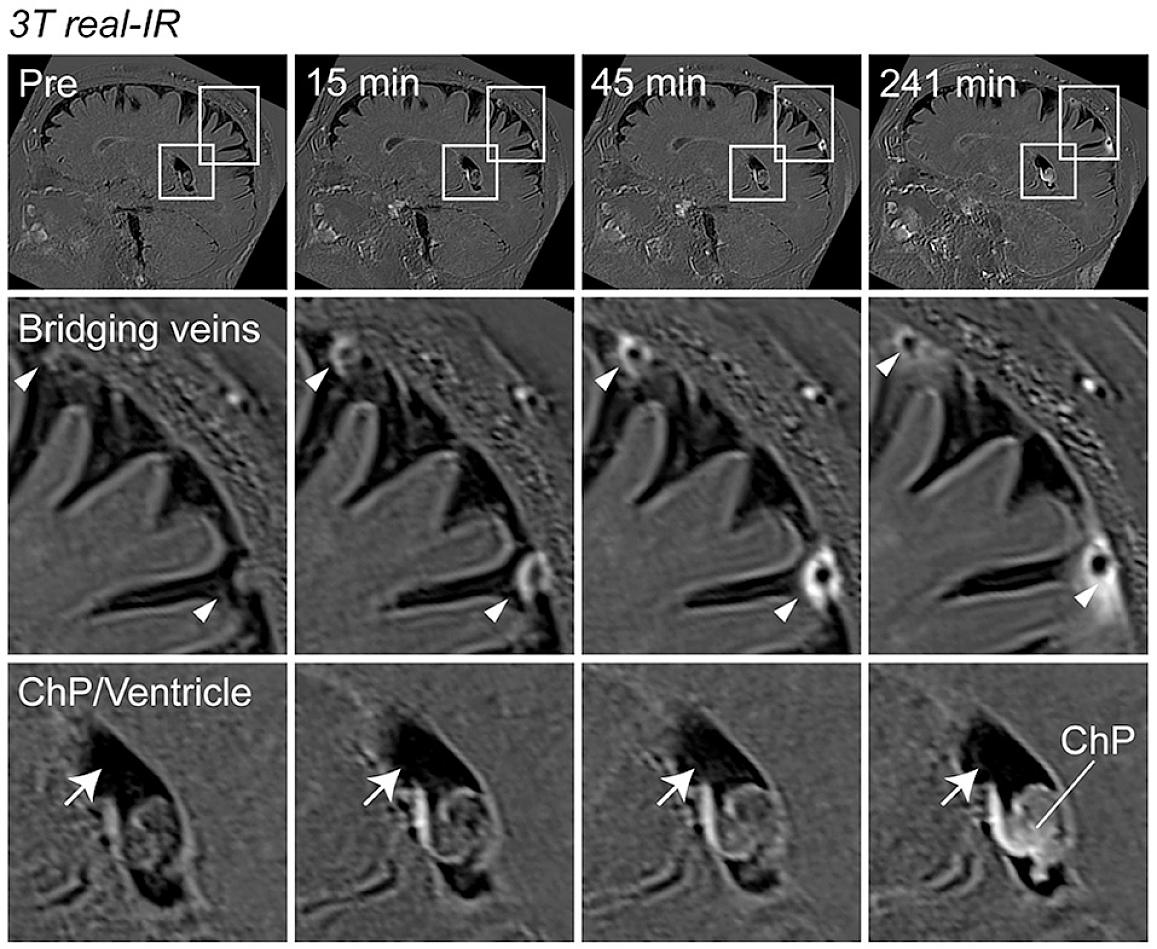
Using MRI, the researchers scanned the brains of a group of healthy volunteers who had received injections of gadobutrol, a magnetic dye used to visualize disruptions in the blood brain barrier or other kinds of blood vessel damage. Large veins are known to pass through the arachnoid barrier carrying blood away from the brain, and these were clearly observed on the MRI scans. As the scan progressed, a ring of dye appeared around those large veins that slowly spread out over time, suggesting that fluid could make its way through the space around those large veins where they pass through the arachnoid barrier on their way into the dura.
Kipnis’s lab was making similar observations in mice. His group injected mice with light-emitting molecules. Like with the MRI experiments, fluid containing these light-emitting molecules was seen to slip through the arachnoid barrier where blood vessels passed through.
Together, the labs found a “cuff” of cells that surround blood vessels as they pass through the arachnoid space. These areas, which they called arachnoid cuff exit (ACE) points, appear to act as areas where fluid, molecules, and even some cells can pass from the brain into the dura and vice versa, without allowing complete mixing of the two fluids. In some disorders like Alzheimer’s disease, impaired waste clearance can cause disease-causing proteins to build up. Continuing the sewer analogy, Kipnis explained the possible connection to ACE points:
“If your sink is clogged, you can remove water from the sink or fix the faucet, but ultimately you need to fix the drain,” he said. “In the brain, clogs at ACE points may prevent waste from leaving. If we can find a way to clean these clogs, its possible we can protect the brain.”
One implication of ACE points is that they are areas where the immune system can be exposed to and react to changes occurring in the brain. When mice in Dr. Kipnis’s lab were induced to have a disorder where the immune system attacks the myelin in their brain and spinal cord, immune cells could be seen around ACE points and even between the blood vessel wall and the cuff cells; this led over time to a breakdown of the ACE point itself. When the ability of immune cells to interact directly with ACE points was blocked, the severity of infection was reduced.
“The immune system uses molecules to communicate that cross from the brain into the dura mater,” said Kipnis. “This crossing needs to be tightly regulated, otherwise detrimental effects on brain function can occur.”
Reich and his team also observed an interesting connection between the participants’ age and the leakiness of ACE points. In older participants, more dye leaked into the surrounding fluid and space around the blood vessels.
“This might point to a slow breakdown of the ACE points over the course of aging,” said Reich, “and this could be consequential in that the brain and immune system can now interact in ways that they’re not supposed to.”
The connection to aging and the disruption of a barrier separating the brain and immune system fits with what has been observed in aging mice and in autoimmune disorders like multiple sclerosis. This newfound link between the brain and immune system could also help explain why our risk for developing neurodegenerative diseases increases as we get older, but more research is needed to confirm this connection.

