

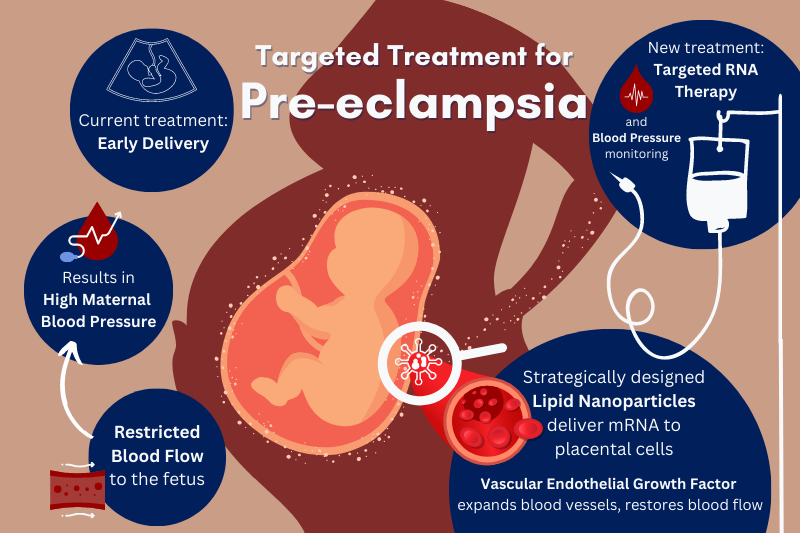
Pre-eclampsia is a leading cause of stillbirths and prematurity worldwide, occurring in 3 – 8 % of pregnancies. A disorder characterized by high maternal blood pressure, it results from insufficient vasodilation in the placenta, restricting blood flow from the mother to the fetus.
Currently, a health-care plan for someone with pre-eclampsia involves diet and movement changes, frequent monitoring, blood pressure management, and sometimes early delivery of the baby. These standards of care address symptoms of the condition, not the root cause, and further perpetuate health inequity.
Now, Penn engineers are addressing this longstanding gap in reproductive health care with targeted RNA therapy.
The COVID vaccines demonstrated how lipid nanoparticles (LNPs) efficiently deliver mRNA to target cells. The success of LNPs is opening doors for a variety of RNA therapies aiming to treat the root causes of illness and disease. However, drug development and health care have consistently neglected a portion of the population in need of targeted care the most – pregnant people and their babies.
In one of the first studies of its kind, published in the Journal of the American Chemical Society, Michael Mitchell, J. Peter and Geri Skirkanich Assistant Professor of Innovation in Bioengineering, and Kelsey Swingle, Ph.D. student in the Mitchell Lab and lead author, describe their development of an LNP with the ability to target and deliver mRNA to trophoblasts, endothelial cells, and immune cells in the placenta.
Once these cells receive the mRNA, they create vascular endothelial growth factor (VEGF), a protein that helps expand the blood vessels in the placenta to reduce the mother’s blood pressure and restore adequate circulation to the fetus. The researchers’ successful trials in mice may lead to promising treatments for pre-eclampsia in humans.

“Current health care for women with pre-eclampsia is lacking,” says Swingle. “Many times, the only thing doctors have been able to do is plan for an early delivery, resulting in premature births which come with associated challenges. This approach is only worsening the health of mothers and their babies in places where premature care is limited.”
Swingle pioneered this study on LNP-assisted RNA therapies for pregnant women and their babies. This is the first time LNP-assisted delivery of mRNA to the placenta has been shown.
“With no previous research to start from, our first challenge was to figure out which LNPs would actually travel to and target the placenta,” says Swingle. “We started by creating a library of LNPs using our knowledge from the work we did on LNP delivery to the liver. It turns out the liver and the placenta are actually very similar. They both receive high blood flow and contain intricate trees of blood vessels.”
By pairing a pregnant person’s natural blood flow to the womb with a highly specific ionizable lipid in the LNP, the research team was able to target and deliver VEGF to placental cells in pregnant mice via a simple tail vein injection.
“The COVID vaccines were administered as intramuscular injections, a shot in the arm,” says Swingle. “This treatment would be administered intravenously. That means a pregnant woman would be able to be treated via a simple, noninvasive, and pain-free IV drip.”
Pregnant women were neglected in the clinical trials for the COVID vaccines, leaving many uncertain about how to safeguard their health and that of their babies. This oversight is not new. The majority of drugs on the market have not been tested in pregnant women and disorders during pregnancy are many times untreatable before birth.
The treatment presented in the groundbreaking study contributes to the health equity of women, a major motivator for Swingle.
“I was inspired to research targeted therapies for maternal and fetal health in the spring of 2021 when people were making decisions about getting the COVID vaccine and pregnant women had questions about safety that we could not answer,” she says. “In the case of pre-eclampsia, many babies are born early, resulting in stunted or abnormal growth and physiological development. A treatment that resolves the issue at the source would allow for higher quality of life and health for both the child and mother over the long term. Addressing this research gap is one way I can be an advocate for women’s health equity.”
The new approach to LNP development for RNA therapies is opening new doors for hard-to-treat diseases. The current study is just one example of where this work is headed.
“Researchers have been doing a lot of work on the mechanisms and movements of drugs into cells, but there is not a lot out there on how therapies can be targeted to treat the root cause of diseases and conditions, particularly during pregnancy.” says Mitchell. “We now have a new LNP platform and can plug and play different RNA therapeutics to develop successful therapies for many pregnancy conditions. This work is leaping off that platform and we are excited to take the next steps.”

