
Additional data from largest head-to-head TAVR trial demonstrated clinical non-inferiority and valve performance superiority of Evolut TAVR Evolut compared to Sapien in challenging patient population
Additional analysis from the SMall Annuli Randomized To Evolut or SAPIEN (SMART) study demonstrated clinical non-inferiority of self-expanding valves (SEV) versus balloon-expandable valves (BEV) in aortic stenosis patients undergoing transcatheter aortic valve replacement (TAVR) and confirmed valve performance superiority over time based on hemodynamics. Data also showed similar positive results in two age groups (< or ≥ 80 years old). The late-breaking results were presented today at the Society for Cardiovascular Angiography & Interventions (SCAI) 2024 Scientific Sessions.
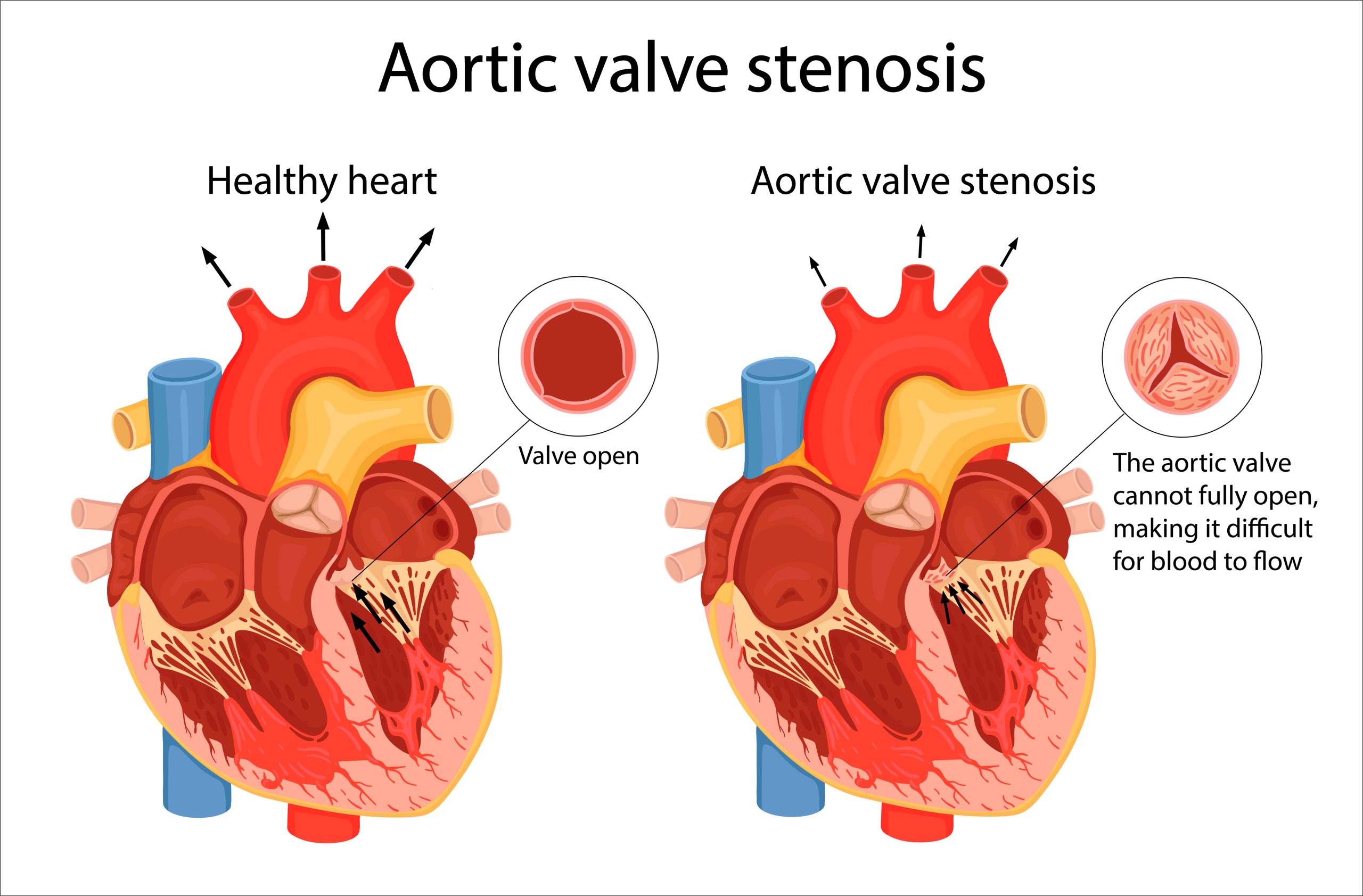
Patients with aortic stenosis (AS) and small aortic annuli (SAA), typically female and underrepresented in clinical trials, are at risk for impaired valvular hemodynamics, or blood flow, and associated adverse cardiovascular outcomes following TAVR. The underrepresentation of women in research and the severity of the condition creates a call for more clinical data.
The SMART trial is an international, prospective, multi-center, randomized (1:1) post-market trial comparing the safety and performance of SEV versus BEV in TAVR patients with symptomatic severe AS and SAA. Eligible patients had an aortic valve annulus area of less than or equal to 430 mm2 by CT and suitable anatomy for transfemoral TAVR with an Evolut PRO/PRO+/FX or a SAPIEN 3/3 Ultra valve. The co-primary endpoints were: a clinical outcome composite of mortality, disabling stroke, or heart failure rehospitalization; and a valve function composite of bioprosthetic valve dysfunction (BVD) through 12 months.
A total of 716 patients (87% women, mean age 80 years) were treated at 83 sites. The SMART trial met both primary endpoints. Compared with BEV, the supra-annular SEV demonstrated non-inferior clinical outcomes and superior valve performance as measured by multiple definitions of bioprosthetic valve dysfunction (BVD) as well as by standard hemodynamics (doppler velocity index [DVI], effective orifice area [EOA], severe prosthesis‐patient mismatch [PPM], mean gradient >20 mmHg) at one year. As an additional analysis, the hemodynamics over time were evaluated and found to be similar between cohorts at baseline and favored SEV compared to BEV at all follow-up time points through 12 months (p<0.001). The results were also consistent in patients with age < or ≥ 80 years. BEV had a BVD difference of 39.8% for patients < 80 years old, and a BVD difference of 27.1% for patients ≥ 80 years old (both p<0.001).
“The SMART trial is the largest, most rigorous trial to date to randomize patients using the two most widely used TAVR devices, and the largest TAVR trial to enroll mostly women. We are excited to reiterate our previously presented findings in front of the interventional cardiologist community with our additional analysis,’’ said Howard C. Herrmann, MD, FACC, MSCAI, John W. Bryfogle Professor of Cardiovascular Medicine and Surgery, Health System Director and Section Chief of Interventional Cardiology, Perelman School of Medicine at the University of Pennsylvania, and lead author of the study. “This confirms the hemodynamic superiority of self-expanding supra-annular valves, which allow for greater options and improved decision-making for clinicians. It was also beneficial to see that this treatment option produced similar positive results for the more active younger patient population.”
Longer-term follow-up of the SMART trial is ongoing.

