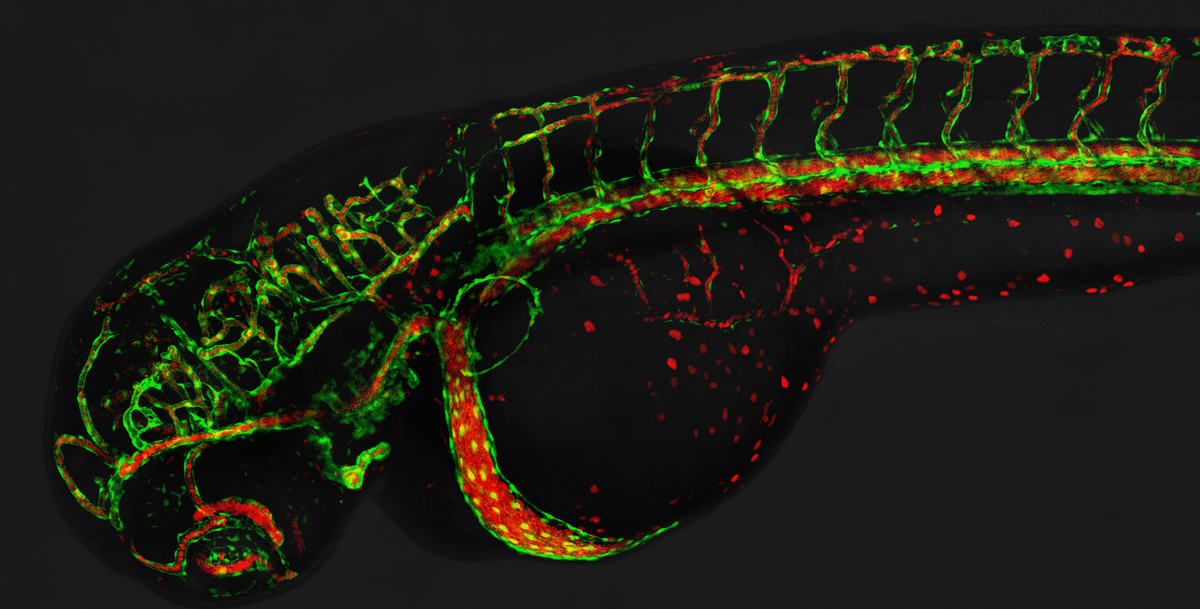

The formation of blood vessels is a complex process involving the interplay of proteins and mechanic forces. In two studies, a research team at the Biozentrum of the University of Basel, Switzerland, has uncovered new mechanisms in blood vessel formation. The team demonstrated how cells interact during vascular lumen formation and the critical role of dynamic forces in this process. These new insights into blood vessel formation may provide potential approaches in the treatment of vascular diseases.
Blood vessels run throughout the entire body, delivering nutrients and oxygen through the circulating blood. During vessel formation, cells first form local lumens, which then fuse to form a continuous tubular network. The junctions between the individual cells must be well sealed and stable to ensure vascular integrity and to prevent leakage.
In two studies, Professor Markus Affolter’s team at the Biozentrum of the University of Basel has now more closely investigated blood vessel formation in zebrafish. The scientists have revealed that the protein Rasip1 plays a key role in the vascular lumen formation. Furthermore, they found that contraction forces are essential drivers of cell interactions, enabling the continuous vascular lumen formation.
Rasip1 plays a key role in vessel formation
In the first study, published in Nature Communications, the scientists demonstrated that the protein Rasip1 is decisively involved in the initial steps of lumen formation, which occurs at the adhesion site between two endothelial cells. Their focus was particularly on the junction between two cells, the adhesion sites.
They observed that this adhesion site is transformed into a hollow space like halves of a nutshell with a sticky adhesive ring on the side. In this process, the protein Rasip1 plays an important role: «It moves the adhesion proteins from the center to the periphery and allows the lumen to inflate in between», says first author Dr. Jianmin Yin.
Tensile forces between the cells ensure correct vessel formation
In a separate study published in «Angiogenesis», the team examined the role of contractile forces regulated by the proteins Heg1 and Ccm1. «We discovered that these contractile forces between the cells are essential. Only when their intensity is precisely regulated do cells interact correctly, enabling proper vessel formation», explains Jianmin Yin.
The scientists have uncovered a mechanism, in which coordinated tensile forces along cell-cell junctions promote coordinated growth of the blood vessels. «We found that tiny forces generated by the rhythmic contraction of cellular structures stabilize cell junctions and thereby help to maintain their shape», says Heinz Georg Belting, who led the study. By selectively activating these forces the researchers were also able to correct defective cell connections. These findings underscore the importance of these forces for a healthy vascular network.
A new understanding of blood vessel formation
Findings on the specific role of dynamic forces and protein regulation deepen our understanding of the process of blood vessel formation. «It is still remarkable to observe this process in the living organism and derive new conclusions », says Belting. «When the balance of forces at the cell junction is disrupted, or proteins misrelate the process, a stable organ structure cannot be formed, resulting in defective blood vessels develop.»
These new research findings could provide a basis for the development of strategies for the treatment of vascular disorders such as aneurysms or peripheral arterial occlusive disease. In the future, the scientists plan to use biophysical methods to examine this process more closely with the aim to better understand the molecular mechanisms of blood vessel formation.

