
Thermography has been a hot topic this year, due to the need for quicker diagnostics to detect and prevent the spread of COVID-19. Noncontact infrared thermometers (NCITs) are currently a primary tool for fever screening, but their widespread use has been prone to inaccuracy. A related medical technology, thermography using infrared thermographs (IRTs), enables increased options for temperature estimation with greater accuracy. Although the use of thermography as a stand-alone detection method for COVID-19 is unlikely to prevent spread, emerging evidence and international consensus suggest that it is indeed possible to use IRTs effectively for detecting elevated body temperatures.
A report published in the Journal of Biomedical Optics provides robust insights for optimizing IRT-based fever screening. The research, performed by Quanzeng Wang and collaborators at the Center for Devices and Radiological Health of the US Food and Drug Administration (FDA), evaluated the use of IRTs under standardized conditions. They examined clinical data for more than 500 demographically diverse individuals, including 47 subjects with elevated oral temperatures (>37.5 °C), correlating facial temperatures to oral under-the-tongue thermometer measurements. The team confirmed the utility of internationally recognized (ISO) standards for obtaining consistently accurate results, and specifically reported the efficacy of ISO methods, including facial area targets.
How do IRTs work?
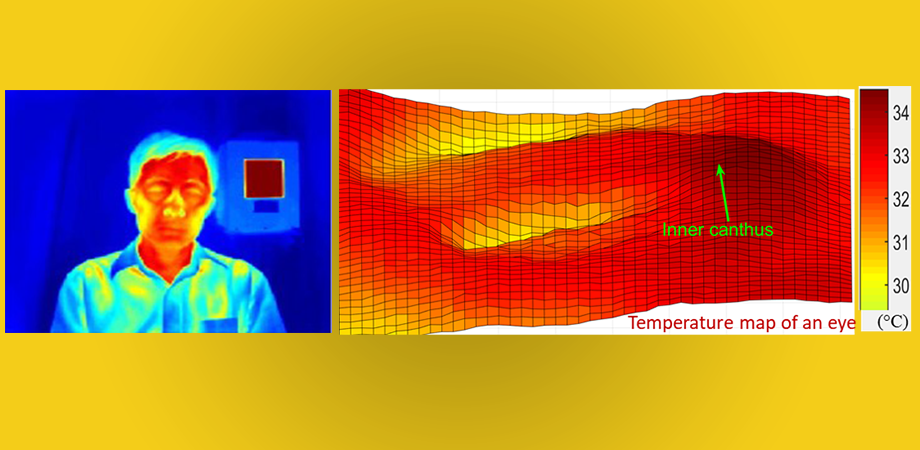
We radiate heat as infrared energy, which changes as we get warmer. IRTs are camera systems that can sense infrared energy, allowing us to “see” the infrared waves. These readings display as a two-dimensional temperature distribution that correlates to temperature levels at the source. A human face typically shows varying temperatures, warmer where the larger arteries are closer to the surface of the skin such as the inner canthi (the eye corners where the tear ducts reside) and temples.

Optimizing IRTs
Like any technology, IRTs are subject to fundamental device and performance variations, which affect overall accuracy when used in medical practice. To minimize such variations, FDA researchers incorporated the ISO guidelines in their experiments (e.g., controlling ambient conditions, requiring individuals to acclimatize for 15 minutes prior to screening, etc.). They reported excellent IRT performance with clear correlations between IRT readings and oral temperature baseline data.
Aside from demonstrating the effectiveness of standardized methods for fever screening, some key insights emerged from the team’s analysis.
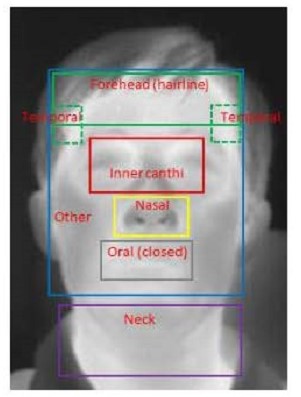
One of the most significant insights was that targeting the full face for temperature screening resulted in greater accuracy than narrowly targeting the inner canthi, as recommended by the ISO guidelines. This insight could be helpful in establishing future directions with these devices, as full-face imaging is much easier to implement since it does not require complex facial feature detection software to identify the inner canthi regions. The next-best performance included the extended inner canthi area that includes but is wider than the inner canthi regions recommended by the ISO guidelines. Targeting the narrow canthi regions produced third-best accuracy.
FDA researchers calculated a series of cutoff levels to optimize the sensitivity and specificity values of the IRT readings. Although there is no uniform consensus on what temperature constitutes a fever, an oral temperature in the range of 37.5°C to 38°C (99.5°F to 100.4°F) is most commonly used in the medical profession as the threshold. The authors caution that determining an appropriate cutoff temperature for real-world fever screening involves complex considerations, such as the likely rate of false positives or negatives, as well as such matters as delay times, staffing, asymptomatic illness, and related costs to health agencies.
IRT-based fever screening
If conducted according to standardized protocols to ensure accuracy, IRT-based fever screening may effectively identify individuals with elevated body temperatures including low-grade fevers (oral temperature?37.5°C) associated with early-stage infections and moderate symptoms. According to Zane Arp, Director of the Division of Biomedical Physics at the FDA, “IRT-based fever screening, especially in regard to addressing COVID-19, should be one element in a multilayered diagnostic process that would include other helpful tools. When used in combination with other diagnostics and medical screening it is a much more useful tool in detecting those who are ill.” For COVID-19 in particular, the FDA has published fact sheets on the proper use and limitations of these systems.
Given the potential value of IRTs for medical diagnostics, optimizing IRT-based fever screening is important, yet there remains much to learn. According to Wang, the FDA team plans further analysis to assess the potential impact of such factors as environmental and intersubject variability.

