
Rare diseases affect fewer than 1 in 2,000 people individually. However, with over 7,000 different types identified, their global impact is significant. In the Asia-Pacific region, approximately 258 million people have a rare disease, the highest number globally, with over 45 million in Southeast Asia alone. This vast number highlights significant challenges in treatment, as the diversity of this patient pool contributes to significant healthcare disparities and increased challenges in clinical trial recruitment. Additionally, within the small patient pool, each patient is different from each other, and individual patients‘ conditions change over time. This emphasises the critical need for accessible and personalised treatments for this population of patients, while highlighting the profound challenges faced in developing treatments for patients with rare diseases.
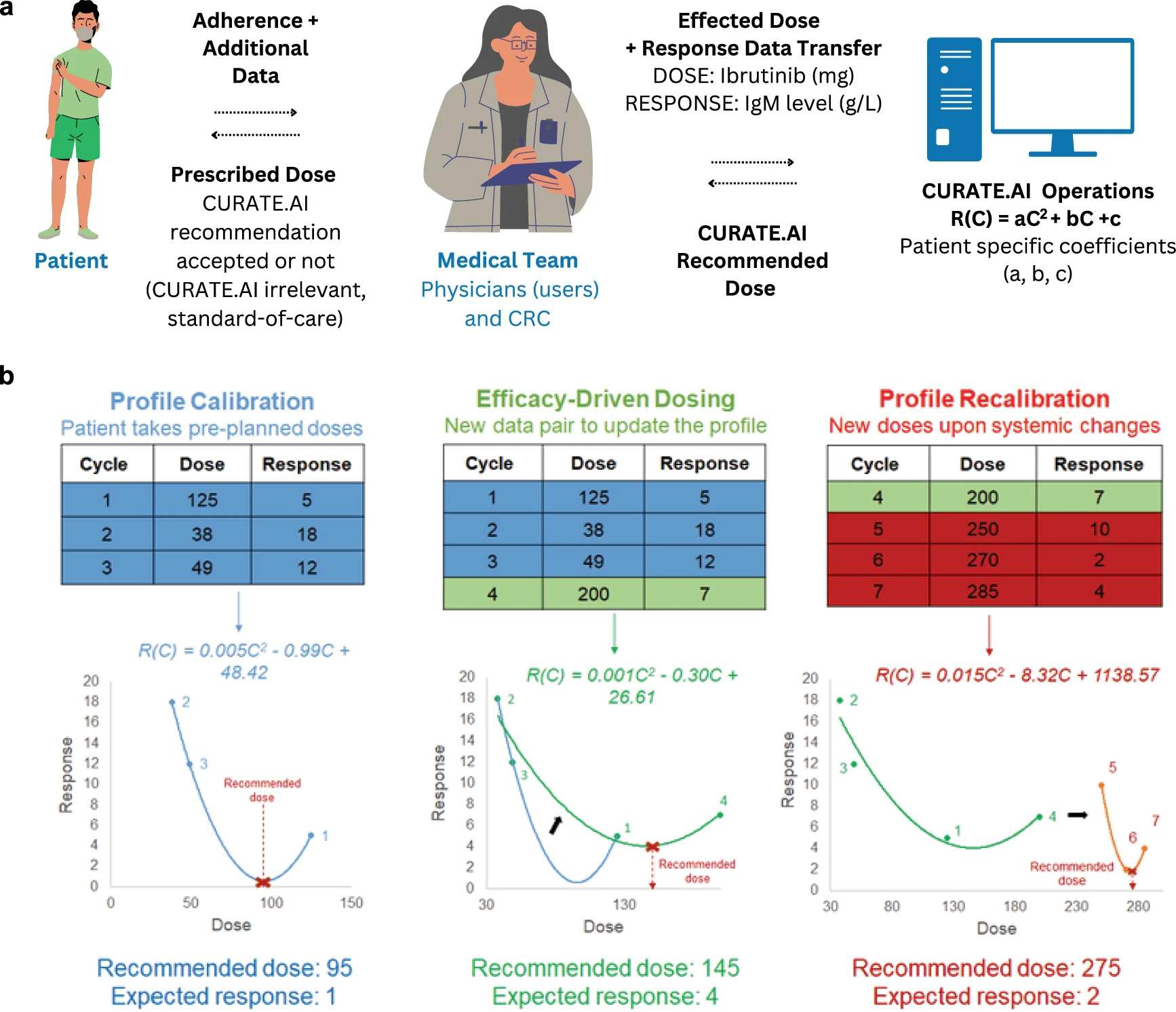
To address the need for effective treatment for rare diseases without using large population data, researchers from the Institute for Digital Medicine (WisDM) at the Yong Loo Lin School of Medicine, National University of Singapore (NUS Medicine) leveraged small amounts of data from a single patient with a rare disease to guide his treatment, with promising results. Co-led by Professor Dean Ho, Director of WisDM at NUS Medicine, the team conducted a clinical trial for the patient who was diagnosed with Waldenström macroglobulinemia—a rare blood disorder affecting approximately three in 1,000,000 people annually—by leveraging an artificial intelligence (AI) derived platform, CURATE.AI. Unlike traditional AI models that depend on large datasets, CURATE.AI utilises small data to dynamically adjust treatment dosages based on individual patient responses. Since the trial began in October 2021, substantial improvement in this patient’s red blood cell levels was observed, and the patient was able to avoid blood transfusions. Importantly, the patient did not suffer serious side effects from the treatment and hospital admissions were minimised.
In the trial, the research team worked with clinicians from the National University Cancer Institute, Singapore (NCIS) to identify drug doses for the patient, based on guidance from the CURATE.AI platform. The drug doses were selected based on the patient’s own responses prospectively, making the treatment strategy a first of its kind. Compared to the overall dose under the standard of care regimen, the trial’s recommended drug doses were lower and well-tolerated by the patient, demonstrating durable control of the disease. As a result, the patient was able to save approximately USD 8,000 (around SGD 10,500) on drug costs over the first two years of the treatment.
The trial with treatment recommended by CURATE-AI is ongoing and is now open for recruitment of new, suitable patients. The results collected from the first two years of the trial are published in the journal NPJ Digital Medicine, a Nature Portfolio journal.
Prof Ho said, “No two patients are alike, and even the same patient can change over time as well. It is essential for treatment to evolve alongside the patient. Our study highlights the effectiveness of using small data to treat extremely rare diseases—addressing the gaps where traditional big data methods fall short, and where large-scale trials are not feasible due to the limited patient population. CURATE.AI’s approach, which tailors treatment using small datasets, offers a practical solution for the urgent and challenging need of developing personalised strategies for rare diseases.” Prof Ho is also Head of the Department of Biomedical Engineering at the NUS College of Design and Engineering, and Director of the NUS N.1 Institute for Health.
Dr. Sanjay de Mel, Senior Consultant in the Division of Haematology, Department of Haematology-Oncology, NCIS, and the clinical lead for the trial, added, “Achieving a good treatment response while minimising side effects is crucial when treating patients with Waldenström macroglobulinemia. Patients may differ significantly in how their bodies handle the treatment and the types of side effects they experience. A personalised approach to medication dosing is therefore required to address this inter-individual variability.”

