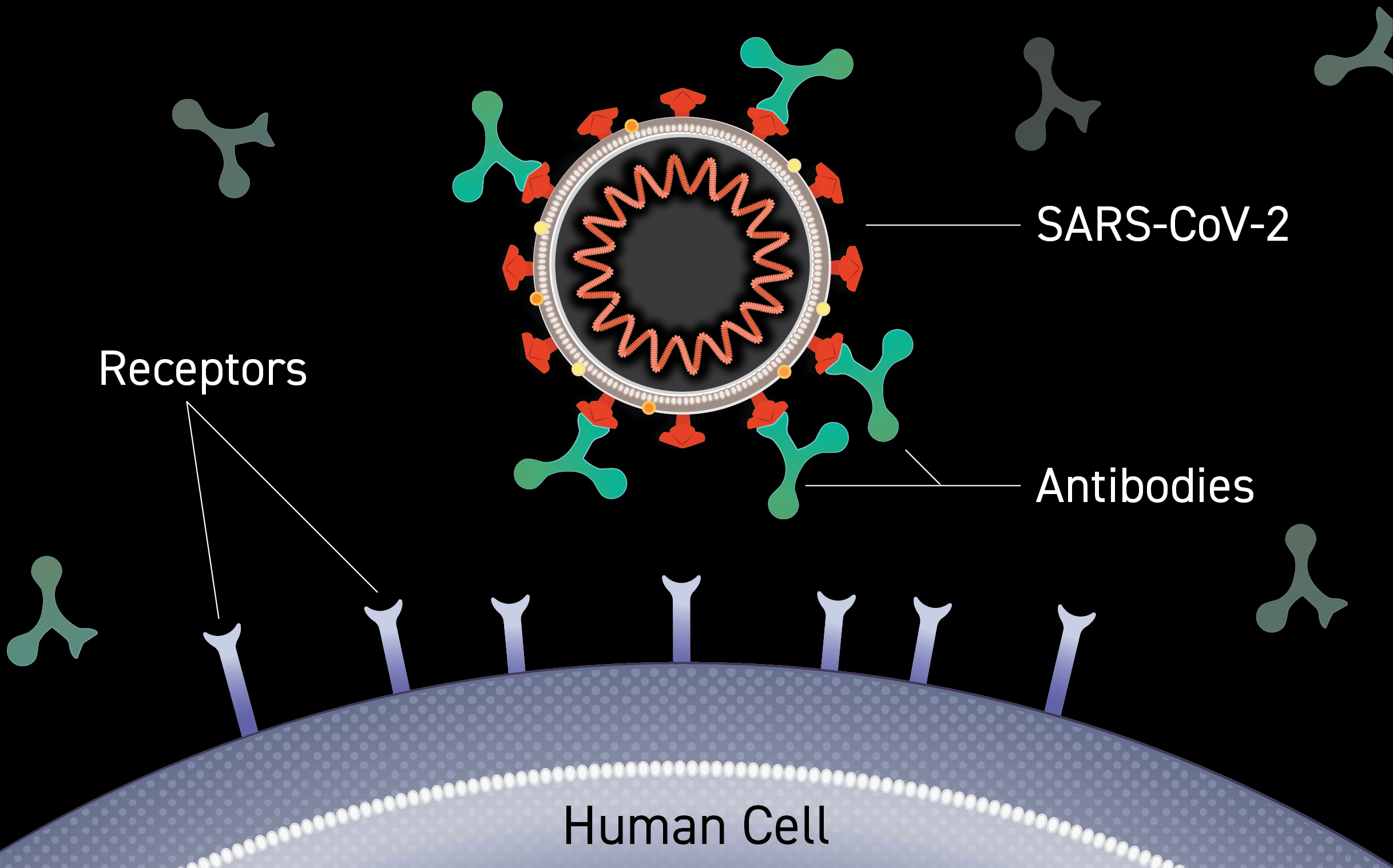

The results of a study led by Northern Arizona University and the Translational Genomics Research Institute (TGen), suggest the immune systems of people infected with COVID-19 may rely on antibodies created during infections from earlier coronaviruses to help fight the disease.
COVID-19 isn’t humanity’s first encounter with a coronavirus, so named because of the corona, or crown-like, protein spikes on their surface. Before SARS-CoV-2 — the virus that causes COVID-19 — humans have navigated at least 6 other types of coronaviruses.
The study sought to understand how coronaviruses (CoVs) ignite the human immune system and conduct a deeper dive on the inner workings of the antibody response. The published findings appear in the journal Cell Reports Medicine.
“Our results suggest that the COVID-19 virus may awaken an antibody response that existed in humans prior to our current pandemic, meaning that we might already have some degree of pre-existing immunity to this virus.” said John Altin, Ph.D., an Assistant Professor in TGen’s infectious disease branch and the study’s senior author.
This knowledge could help researchers design new diagnostics, evaluate the healing powers of convalescent plasma, develop new therapeutic treatments, and — importantly — help design future vaccines or monoclonal antibody therapies capable of protecting against mutations that may occur in the COVID-19 virus.
The researchers used a tool called PepSeq to finely map antibody responses to all human-infecting coronaviruses. PepSeq is a novel technology being developed at TGen and NAU that allows for the construction of highly diverse pools of peptides (short chains of amino acids) bound to DNA tags. When combined with high-throughput sequencing, these PepSeq molecule pools allow for deep interrogation of the antibody response to viruses.
“The data generated using PepSeq allowed for broad characterization of the antibody response in individuals recently infected with SARS-CoV-2 compared with those of individuals exposed only to previous coronaviruses that now are widespread in human populations,” said Jason Ladner, Ph.D., an Assistant Professor at NAU’s Pathogen and Microbiome Institute, which combines the academic genomic research focus of NAU and the translational genomics capacity of TGen. Dr. Ladner is the study’s lead author.
Besides SARS-CoV-2, researchers examined the antibody responses from two other potentially deadly coronaviruses: MERS-CoV, which caused the 2012 outbreak in Saudi Arabia of Middle East Respiratory Syndrome; and SARS-CoV-1, the first pandemic coronavirus that caused the 2003 outbreak in Asia of Severe Acute Respiratory Syndrome. All three are examples of coronaviruses that infect animals, but evolved to make people sick and became new human pathogens.
In addition to characterizing antibodies that recognize SARS-CoV-2, they also examined the antibody responses of four older coronaviruses: alphacoronavirus 229E; alphacoronavirus NL63; betacoronavirus OC43; and betacoronavirus HKU1. These so called “common” coronaviruses are endemic throughout human populations, but usually are not deadly and cause mild upper respiratory infections similar to those of the common cold.
By comparing patterns of reactivity against these different coronaviruses, the researchers demonstrated that SARS-CoV-2 could summon immune system antibodies originally generated in response to past coronavirus infections. This cross-reactivity occurred at two sites in the SARS-CoV-2 Spike protein; the protein on the surface of virus particles that attaches to ACE2 proteins on human cells to facilitate cell entry and infection.
“Our findings highlight sites at which the SARS-CoV-2 response appears to be shaped by previous coronavirus exposures, and which have potential to raise broadly-neutralizing antibodies. We further demonstrate that these cross-reactive antibodies preferentially bind to endemic coronavirus peptides, suggesting that the response to SARS-CoV-2 at these regions may be constrained by previous coronavirus exposure,” said Dr. Altin, adding that more research is needed to understand the implications of these findings.
The findings could help explain the widely varying reactions COVID-19 patients have to the disease; from mild to no symptoms, to severe infections requiring hospitalization, and often resulting in death. It’s also possible that differences in the pre-existing antibody response identified by this study could help to explain some of the difference in how severely COVID-19 disease manifests in old versus young people, who will have different histories of infections with the common coronaviruses.
“Our findings raise the possibility that the nature of an individual’s antibody response to prior endemic coronavirus infection may impact the course of COVID-19 disease,” Dr. Ladner said.

