
As widely-anticipated decisions about COVID-19 vaccine boosters roll out from U.S. agencies today, insights from an independent study underscore why boosters are important for all adults.
Antibody levels after receiving the Pfizer-BioNTech COVID-19 vaccine vary by age and sex, but across the board, antibody levels dropped significantly within six months, according to an ongoing study led by Texas Biomedical Research Institute (Texas Biomed) and the University of Verona in Italy.
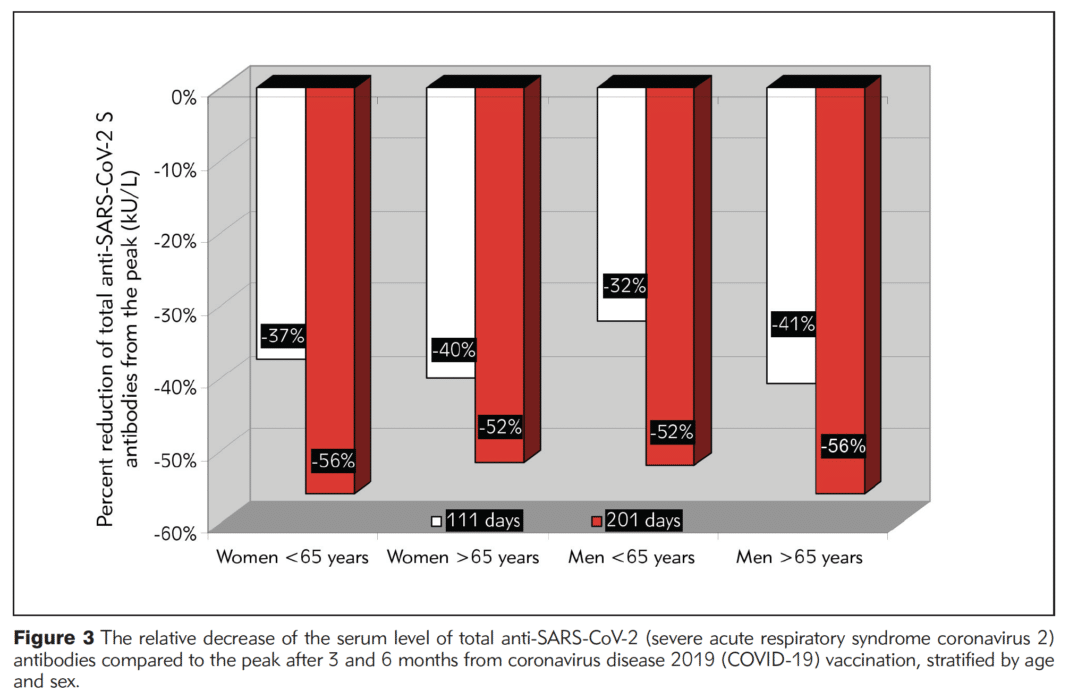
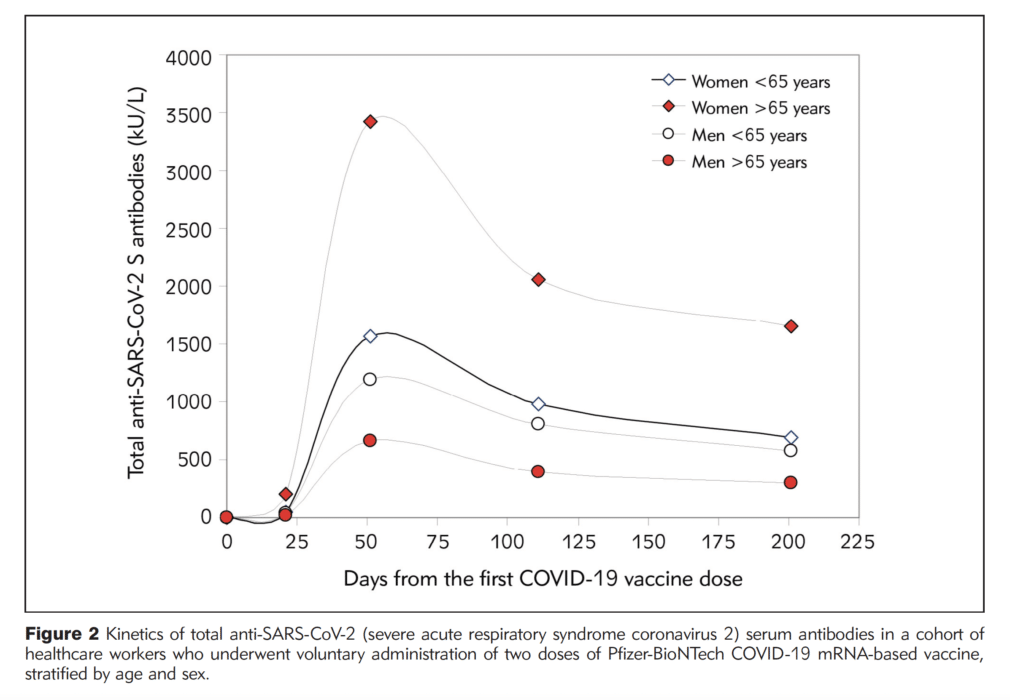
The study found total antibody levels against SARS-CoV-2 varied between age groups and between men and women. Specifically, individuals under the age of 65 had more than twice the level of antibodies than individuals 65 years and older throughout the six months following vaccination. Women had higher antibody levels than men, especially women under the age of 65. Importantly, however, by the six-month mark, antibody levels had decreased by more than 50% from peak levels for everyone in the study.
“While we see how well vaccines have helped keep people out of the hospital and prevent life-threatening disease, antibody levels are quickly declining in all persons regardless of age and sex,” says Brandon Michael Henry, MD, a physician scientist and postdoctoral researcher at Texas Biomed who co-led the study with collaborators in Italy. “Our study provides additional evidence that booster shots for all adults will be important to keep antibody levels up so we can continue to mount an effective immune response against COVID-19 infection and prevent COVID-19 fatalities.”
The findings are based on a group of 787 healthcare workers in Verona, Italy who received two doses of the Pfizer-BioNTech COVID-19 vaccine. They ranged in age from 21 to 75. Their antibody levels were measured before vaccination, after the second dose, and at one, three and six months after the second shot. Henry presented the research at the 9th annual Vaccine Development Center of San Antonio Conference on Nov. 11. The paper has been accepted for publication in the Journal of Medical Biochemistry (preprint here).
Henry and collaborators theorize that the significant sex differences have to do with hormones. Testosterone, which is higher in men, naturally suppresses the immune system, whereas estrogen, which is higher in women, is known to amplify immune responses. Also, some genes that code for certain immune proteins are on the X chromosome, and since women have two X chromosomes, this might help increase immune activity.
“Normally, only one X chromosome is active and the other is mostly deactivated, but there is evidence that immune-related genes stay active on that redundant chromosome and help boost immune responses in women,” Henry says.
However, women, regardless of age, still saw their antibody levels drop by more than 50% from the peak by six months post vaccination.

Henry has also led systematic reviews that show similar results for age and gender. He and colleagues developed a method to standardize research results for antibody levels, by looking at the percentage change in antibody levels, across 32 studies encompassing more than 5,000 people.
“We have observed throughout the pandemic more older people and men suffer the worst consequences of COVID-19,” Henry says. “These studies point to weaker immune responses against SARS-CoV-2 as a contributing factor to this phenomenon.”
Henry stressed that the decline in antibody level does not mean that the vaccines are not effective. Different types of antibodies play different roles in preventing a mild breakthrough infection versus severe disease. The antibodies that help prevent severe disease appear to continue to be effective in most groups even if present at a lower level, which is why vaccination is important. However, as these antibodies will continue to decline with time, booster doses can help maintain adequate levels of these lifesaving antibodies.
On Friday, Nov. 19, the U.S. Food and Drug Administration (FDA) approved boosters for all adults. The Centers for Disease Control and Prevention (CDC) is expected to issue its formal recommendation soon. Previously, the FDA and CDC approved and recommended boosters for certain groups of people. Several states have already moved forward with offering boosters for all adults six months after vaccination.

