
The community of microbes living in and on our bodies may be acting as a reservoir for antibiotic resistance, according to new research from the Earlham Institute and Quadram Institute in Norwich.
The use of antibiotics leads to ‘collateral damage’ to the microbiome, ramping0 up the number of resistance genes being passed back and forth between strains in the microbiome.
The findings also suggest these genes spread so easily through a population that, regardless of your own health and habits, the number of resistance genes in your gut is heavily influenced by national trends in antibiotic consumption.
The rise of antimicrobial resistance (AMR) among human pathogens is widely seen as one of the most serious threats to global health in the coming decades. AMR is already believed to be contributing to tens of thousands of deaths in Europe each year.
Tracking the emergence and spread of genes that help these pathogens to shrug off antibiotics has generally been limited to samples taken from infected individuals. The majority of microbes living in the human body, however, are not pathogenic.
The human microbiome is a complex and dynamic community of millions of species of microbes, primarily living in the gut and coexisting with us. Microbiomes play an important role in health and disease, with the gut microbiome known to help with the digestion of food and the development of our immune system.
Professor Chris Quince, author of the research at the Earlham Institute and Quadram Institute, said: “Even a healthy individual, who hasn’t taken antibiotics recently, is constantly bombarded by microbes from people or even pets they interact with, which leads to resistance genes becoming embedded in their own microbiota.
“If they exist in a population with a heavy burden of antibiotic consumption, it leads to more resistance genes in their microbiome.”
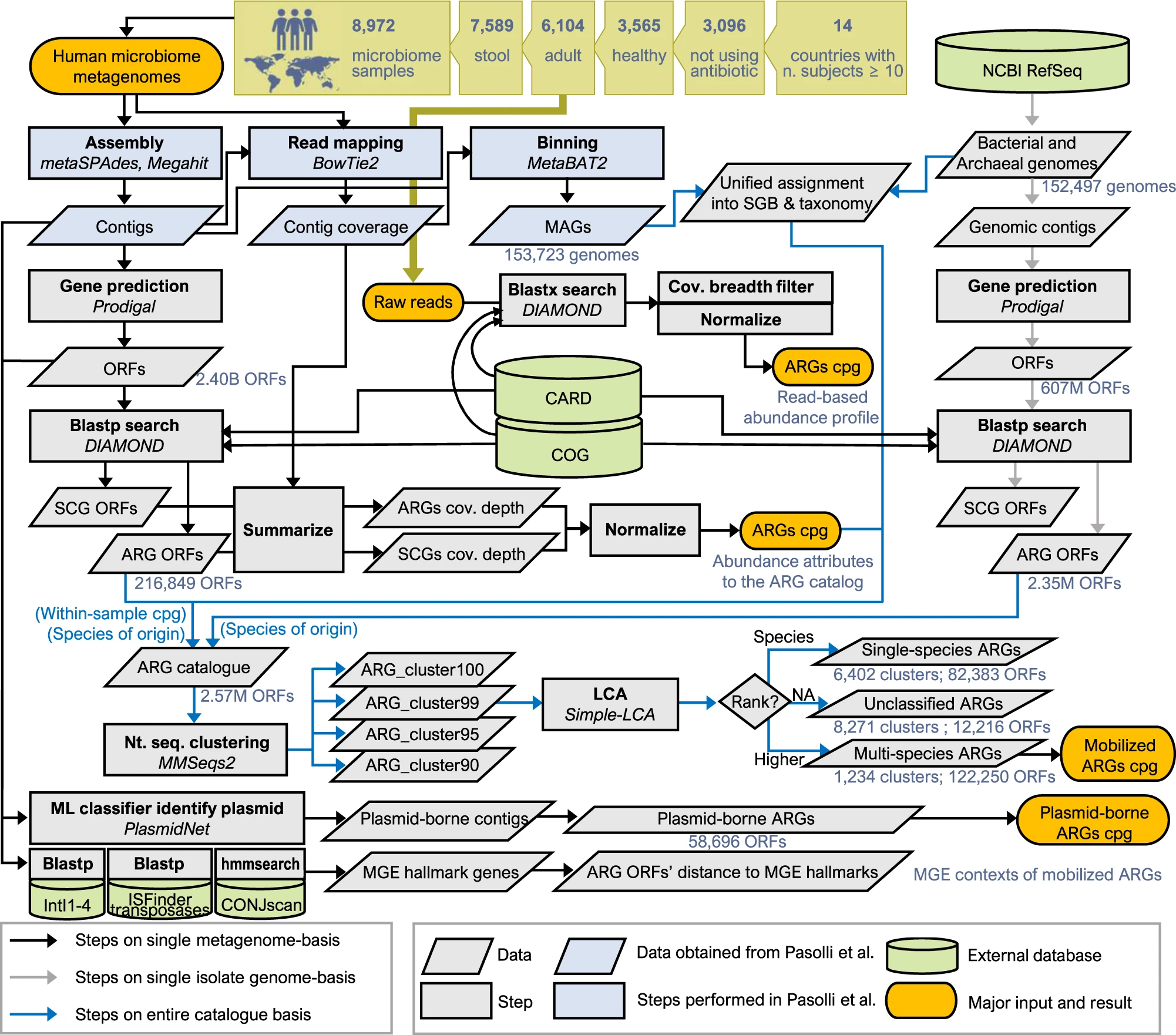
To better understand the impact of antimicrobials on the gut microbiome, researchers at the Earlham Institute and Quadram Institute in Norwich, together with collaborators in the Republic of Korea, analysed over 3,000 gut microbiome samples, collected from healthy individuals across 14 countries.
They then compared the resistance genes identified in samples to those found in large genome collections in order to understand the movement of AMR genes between microbe and pathogen species.
“We deliberately focussed on samples from healthy people, or at least those we could be confident weren’t taking antibiotics,” explained Professor Quince. “We needed to see the gene profile in the gut microbiome without the influence of any antimicrobials.”
They carefully catalogued and recorded the number of antimicrobial resistance genes found in the samples by comparing data to the Comprehensive Antibiotic Resistance Database, a public health resource where resistance genes are documented.
The team identified a median of 16 AMR genes per stool sample analysed. They also found the median number of genes varied across the 14 countries for which they had data.
For example, they saw a five-fold variation in median resistance levels between the lowest in the Netherlands and the highest in Spain.
Using World Health Organization and ResistanceMap data, the team were able to show a strong correlation between the frequency of resistance genes present in a country and national antibiotic consumption levels.
“We found that, in countries where antibiotics are taken more regularly, their populations also have higher numbers of resistance genes in their gut microbiome,” said Professor Quince.
The reason this collateral damage is such a major problem is that microbes are constantly sharing genes with each other. Known as horizontal gene transfer, this process helps AMR genes to spread back and forth between species.
“Our bodies are continually importing and exporting microbes and pathogen strains,” explained Professor Quince. “These strains are themselves passing genes back and forth, which means the challenge of AMR has to be tackled at both the micro and macro level.
“Given our complex relationship with microbes, we need to do more research to understand how we maximise the benefits and minimise the risks when it comes to guiding treatment decisions and developing new medicines.”
Professor Falk Hildebrand, research author at the Quadram Institute and Earlham Institute, said: “We’ve known for some years that antimicrobial resistance genes can spread incredibly fast between gut bacteria.
“This study is so important because it can, for the first time, quantify the impact national antibiotic usage has on our commensal bacteria, as well as giving us insights into the common types of resistance we can expect to evolve.”
The researchers plan to carry out further research – and encourage others to – in order to investigate the relationship in more countries and inform public health strategies.

