GLP-1 Agonist Use and Side Effects in the United States
GLP-1 agonists are a class of medications that are prescribed to manage blood sugar for those with type 2 diabetes and to treat obesity. These drugs work by imitating glucagon-like peptide-1 (GLP-1), a growth hormone that triggers insulin release, reduces blood sugar levels, slows the progress of food along the intestinal track, and stimulates parts of the brain that control craving and satiety. The most commonly prescribed GLP-1 agonist is semaglutide, which has gained popularity in recent years after the Food and Drug Administration (FDA) approved it for the treatment of diabetes in 2017 (under the brand name Ozempic), for the treatment of obesity in 2021 (under the brand name Wegovy), and for reducing cardiovascular disease risk in 2024 (Wegovy). Since 2020, the number of prescriptions made for these drugs has more than tripled (Jain, 2023).
Given the recency of FDA approval and the rapid speed at which these drugs are penetrating the market, research on their use and their side effects at the population level is in its infancy. In April 2023, KFF fielded a survey to a nationally representative sample of 1,479 adults in the United States from the SSRS Opinion Panel to obtain population estimates of GLP-1 agonist knowledge, attitudes, and use (Montero et al., 2024). The KFF survey findings show that 12 percent of adults reported having ever taken a GLP-1 agonist (Montero et al., 2024).
In our brief report, we update this estimate using data from a survey fielded to a nationally representative sample of 8,793 adults from the RAND American Life Panel. Members of the panel are drawn using probability sampling methods, and the data are weighted to reflect the demographic diversity of the U.S. population. The survey was fielded in April and May 2025, approximately two years after the KFF study. Our more-recent survey data allow us to examine whether GLP-1 use rates have changed since 2023. Additionally, we highlight age and sex differences, key demographic dimensions not fully explored in the KFF study. Finally, we provide the first population-level estimates of side effects experienced by those who have taken GLP-1 agonists. Readers should keep in mind that the data presented here are self-reported; therefore, they may not comport with other possible estimates based on diagnoses from health care professionals or on prescription data. All of the data presented in the figures, as well as standard errors, confidence intervals, and sample sizes, can be found in the appendix.
One in Five Women Between the Ages of 50 and 64 Has Taken a GLP-1 Agonist
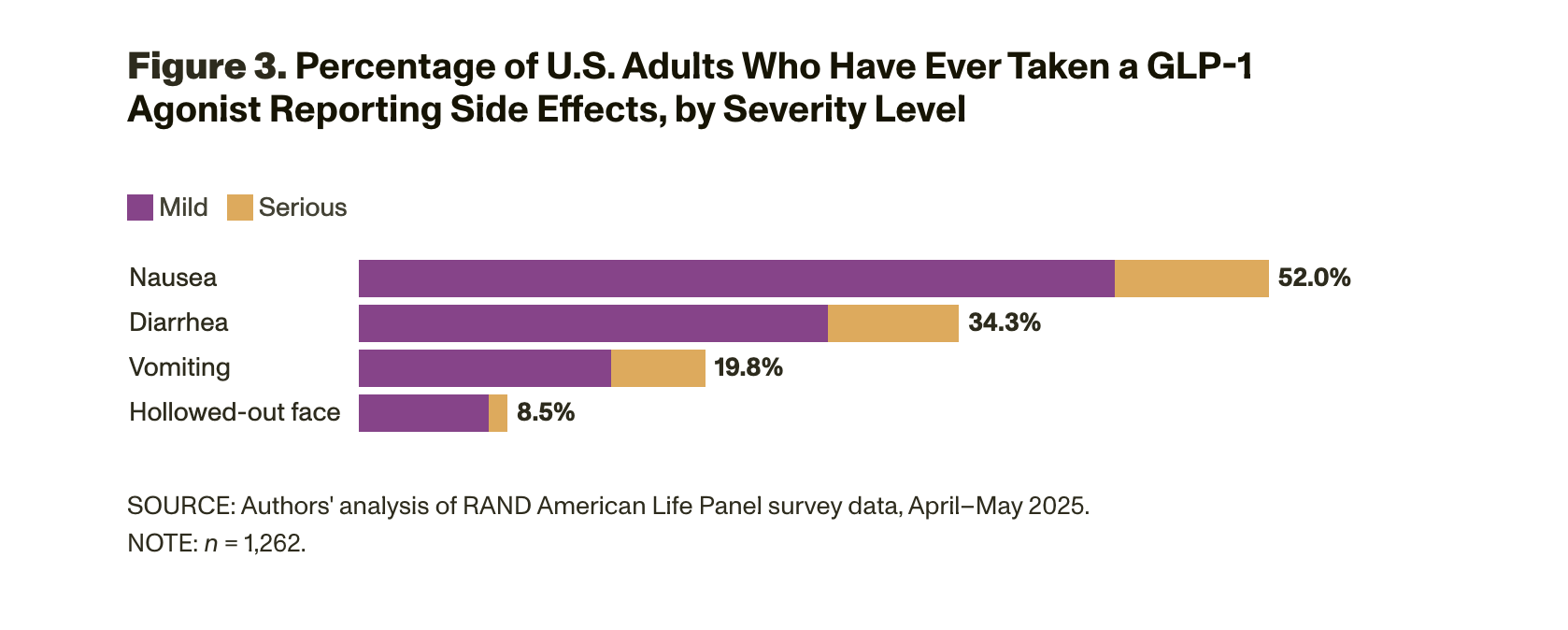
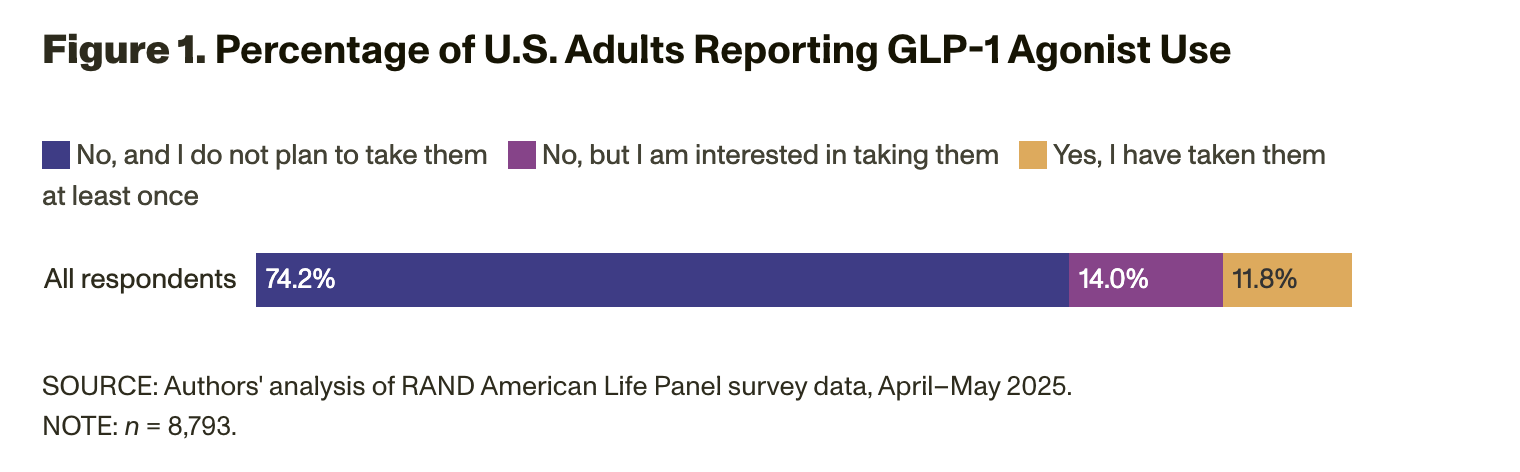
We asked RAND American Life Panel members to report whether they are taking or have ever taken a GLP-1 agonist. Specifically, survey participants in our sample were asked the following question: “You may have heard about a class of prescription drugs used both for weight loss and to treat diabetes or to reduce heart disease risk. These include drugs like Ozempic®, Wegovy®, Mounjaro®, Zepbound®, Rybelsus®, Trulicity®, semaglutide, and others. These drugs are sometimes referred to as GLP-1 agonists. Are you currently taking or have you ever taken one of these drugs to lose weight or treat a chronic condition such as diabetes or heart disease?” Panel members were given three response options: (1) “Yes, I have taken them at least once”; (2) “No, but I am interested in taking them”; and (3) “No, and I do not plan to take them.” Figure 1 shows that 11.8 percent of adults reported using GLP-1 agonists in 2025 — about the same percentage of users reported in 2023 by KFF. This finding suggests that overall rates of use in the population have held steady over the past two years. Our data show that another 14.0 percent of survey respondents expressed an interest in taking GLP-1 agonists.

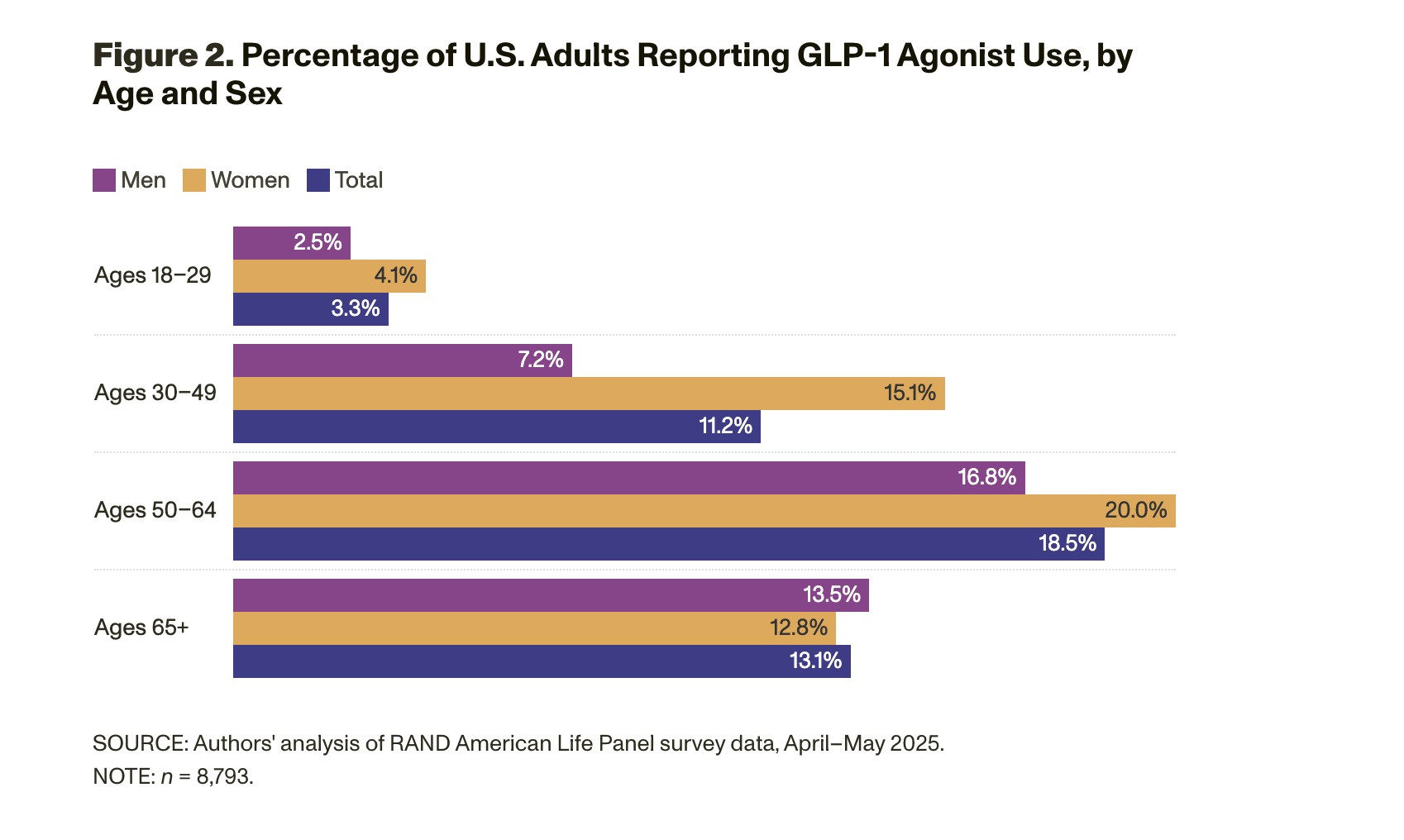

Those panel members who reported GLP-1 agonist use were then asked whether they experienced any of four known side effects while taking the drug: nausea, diarrhea, vomiting, and/or hollowed-out face. Figure 3 shows the overall prevalence of these side effects among our survey sample, with distinctions made as to whether the side effect was mild or severe. The most common side effects reported were nausea and diarrhea: About half of adults who use GLP-1 agonists reported having experienced nausea, and about one-third of adults who use GLP-1 agonists reported having experienced diarrhea. Additionally, about one-fifth of adults who use GLP-1 agonists reported having experienced vomiting. The majority of those who reported having side effects noted that they are mild and not serious.